UNIT 11
Barometers and manometers
Consider a container full of water as in the picture below. Is there a pressure at the top surface of the liquid?
The air in the atmosphere is a fluid and it exerts a pressure on the surface of the water.
Consider a tube filled with mercury. When the tube is turned over, into a container of mercury, the mercury in the tube flows out and then stops, as in the picture below.
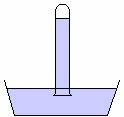
If the atmosphere is exerting a pressure on the surface of the mercury, and the pressure in the container is the same at a particular level, then at a line drawn at the level where the atmosphere meets the mercury, as in the diagram below, the upward pressure due to the mercury below the line must equal the downward pressure due to the mercury in the tube above the line and this must equal the pressure of the atmosphere downwards. Since we can calculate the pressure due to the mercury in the tube, we can use this instrument to measure atmospheric pressure. It is called a barometer.
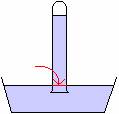
Suppose the height of the mercury in the tube above the level where the atmosphere meets the mercury is 760mm high. The density of mercury is 13.6 ´ 103 kg/m3. We can calculate the atmospheric pressure using the hydrostatic equation.
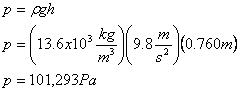
When measuring pressure, people sometimes report only the height of the mercury in the tube, so you will hear atmospheric pressure referred to as 760mm of mercury. To get the pressure in standard units, the pressure must still be calculated as above.
Another instrument similar to a barometer is a manometer. It simply consists of a U-shaped tube filled with liquid, as in the diagram below.
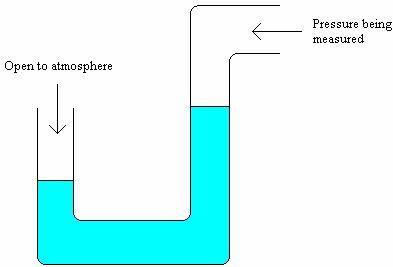
If both ends of the tube are open to the atmosphere the level of the liquid will be the same in each side of the tube. If one end of the tube is open to the atmosphere and the other end of the tube is open to a fluid at a different pressure, then the level of the liquid will not be the same on each side, depending on the pressure of the fluid.